Pharmaceutical Project Management Software
Ensure compliance, standardize workflows, and accelerate product development







Manage pharma projects with structure and confidence
Pharmaceutical organizations manage projects in highly regulated, high-risk environments. From early research initiatives to development programs and operational improvements, success depends on clear governance, reliable data, and consistent execution.
Cerri Project provides a centralized project management platform designed to support structured processes, controlled collaboration, and full visibility across life-sciences initiatives.
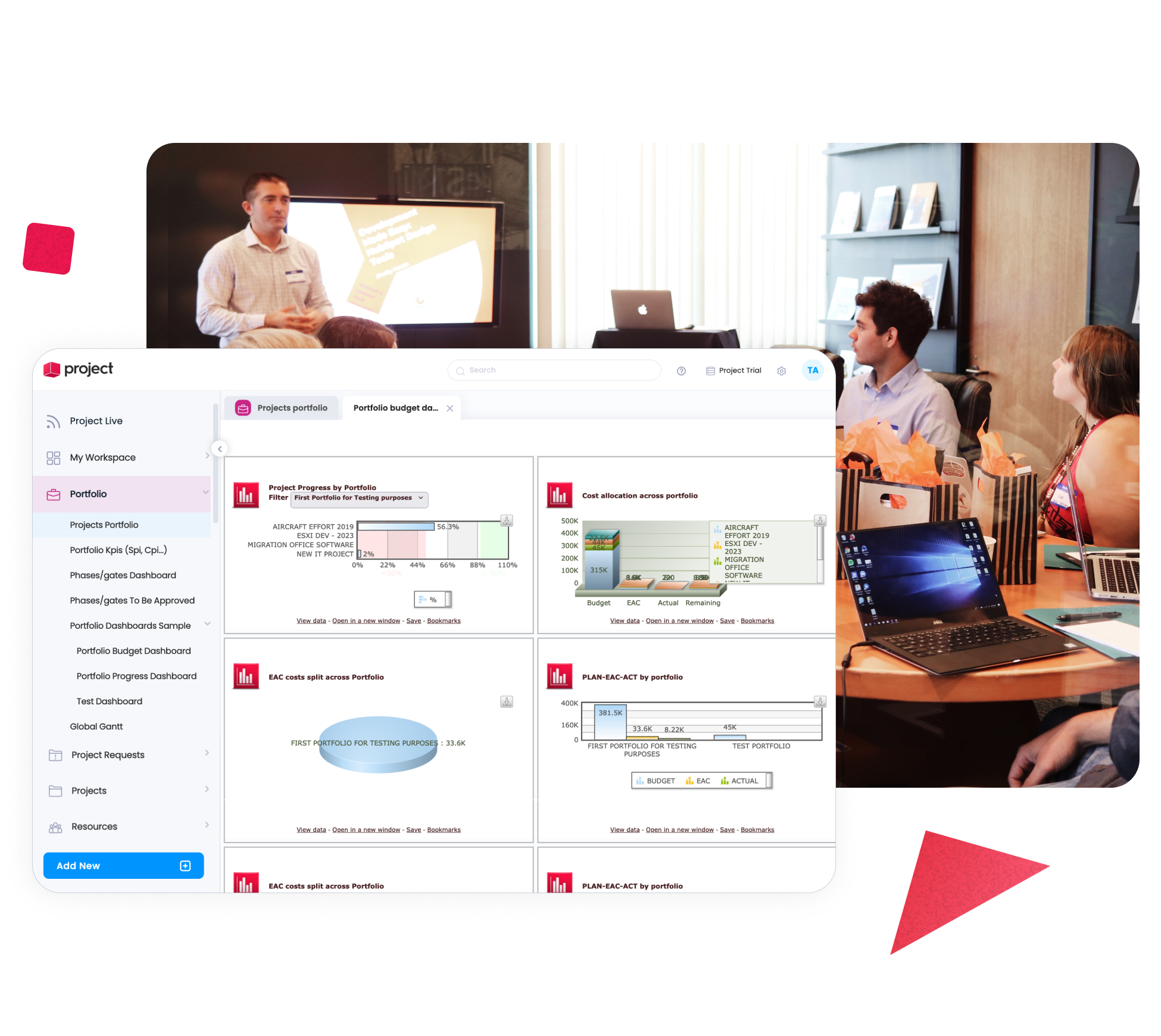
Align Project Portfolios with Strategic Priorities
Organizations operating in pharma must balance innovation, compliance, capacity, and cost. Cerri Project enables portfolio-level visibility across research, development, and operational projects – helping decision-makers prioritize initiatives, assess trade-offs, and align investments with business objectives.
Support Gated and Phase-Based Execution
Projects in regulated life-sciences environments often rely on structured phases and formal decision points. Cerri Project supports configurable workflows that reflect gated processes, reviews, and approvals, ensuring that initiatives progress in a controlled and traceable manner while remaining adaptable to different project types.
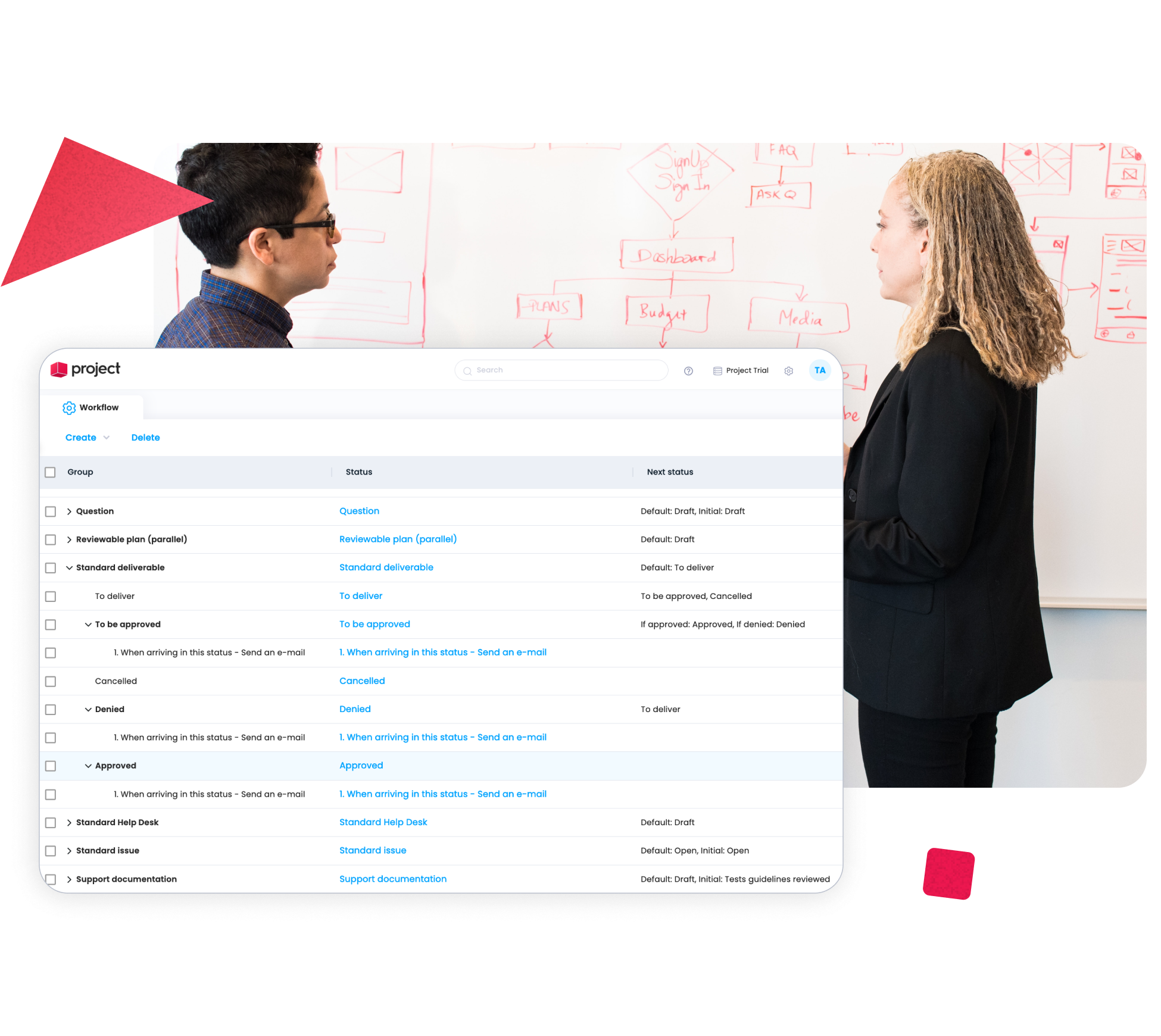
Standardize Processes Across Teams and Departments
Consistency is critical in pharma environments. With Cerri Project, organizations can standardize project structures, workflows, and reporting across departments such as R&D, quality, regulatory, manufacturing, and IT – reducing variability and improving coordination across teams.
Control Documentation and Ensure Traceability
Documentation plays a central role in pharma compliance. Cerri Project provides a centralized repository where project documents are linked directly to tasks, deliverables, and approvals. Role-based access and version tracking help teams maintain traceability and control throughout the project lifecycle.
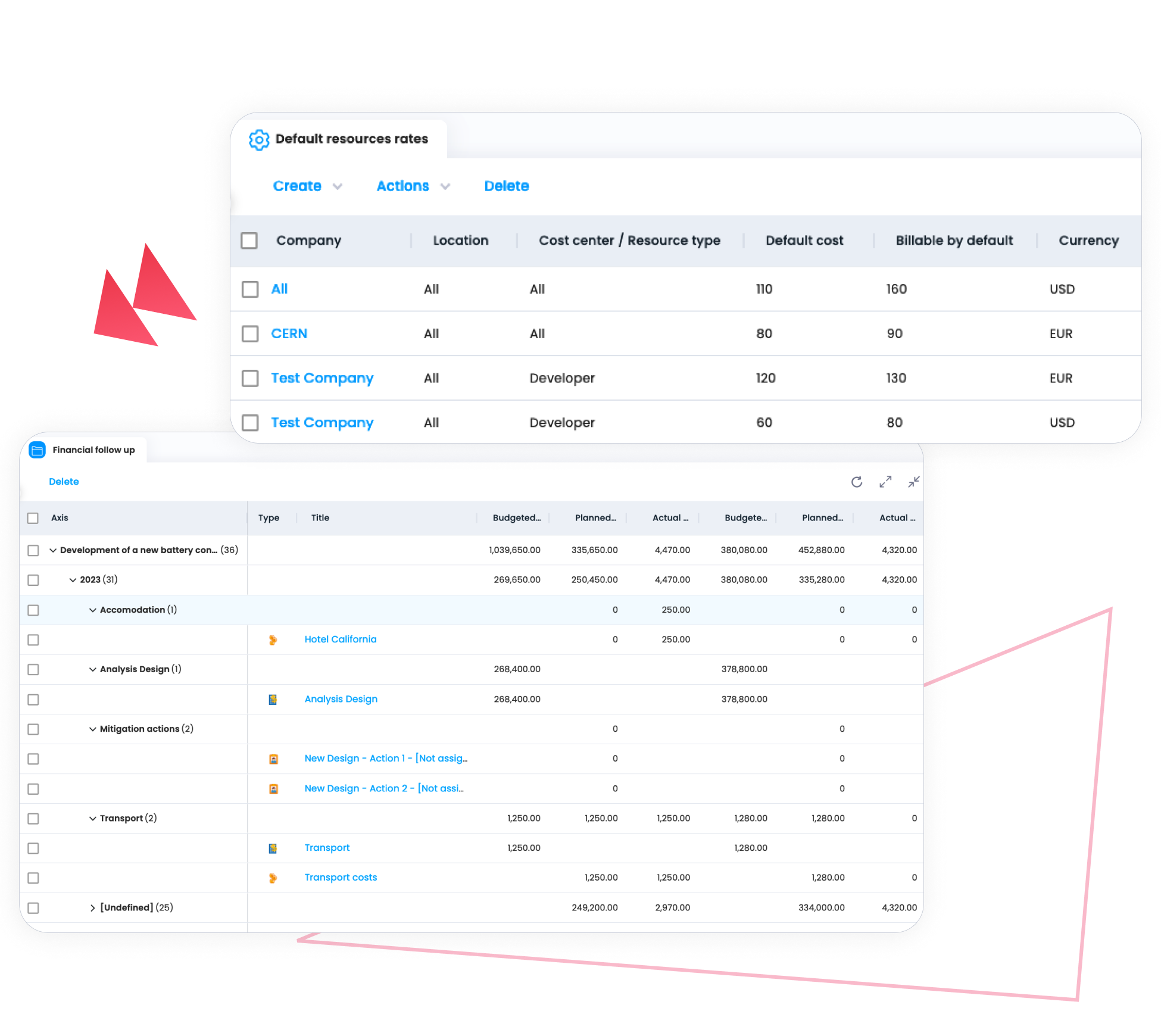
Improve Visibility into Costs and Resources
Projects in the pharmaceutical sector often involve long timelines and specialized expertise. Cerri Project helps organizations plan and monitor budgets, effort, and resource capacity across projects – allowing teams to anticipate constraints, manage workloads, and maintain financial control.
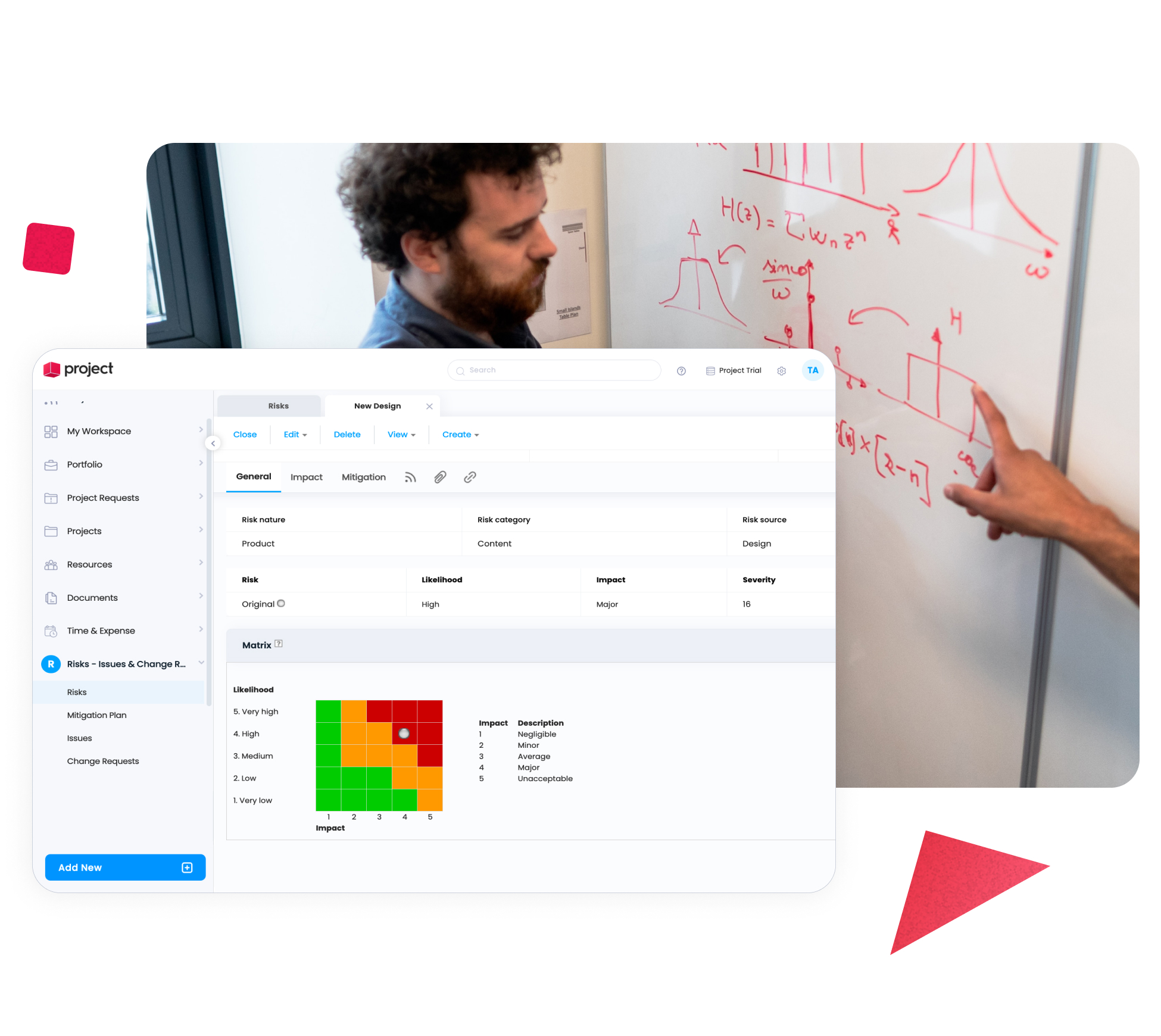
Manage Risks and Changes Proactively
Change and risk are unavoidable in pharma projects. Cerri Project enables teams to document risks, track issues, and manage change requests with clear visibility into their impact – supporting proactive decision-making and reducing downstream disruption.
Secure Deployment for Sensitive Project Data
Organizations in pharma and life sciences often require strict data hosting and access controls. Cerri Project offers flexible deployment options, including on-premise and private cloud environments, allowing organizations to meet internal policies and regulatory expectations while maintaining full control over their data.

Explore more solutions for pharmaceutical project success
Manufacturing project management goes beyond tracking tasks – it’s about driving innovation, optimizing workflows, and ensuring on-time delivery. Discover how Cerri Project supports key areas of your operations:
New Product Development (NPD)
Accelerate time to market and manage complex product launches efficiently.
Explore NPD Solutions
Gantt Chart Scheduling
Plan and execute manufacturing projects with precision using interactive Gantt charts.
Explore Gantt Chart features
Stage and Gate Process
Standardize approvals and improve project success with structured phase reviews.
Explore Stage Gate features
Optimize your business processes
with Cerri Project
with Cerri Project
Cerri Project offers a wide range of project management tools to help you maximize ROI and achieve your full productivity potential.
Here are some.
Here are some.

Best project management software for the pharmaceutical industry
START OPTIMIZING YOUR OPERATIONS












 Task Management
Task Management 




















 Customization
Customization
